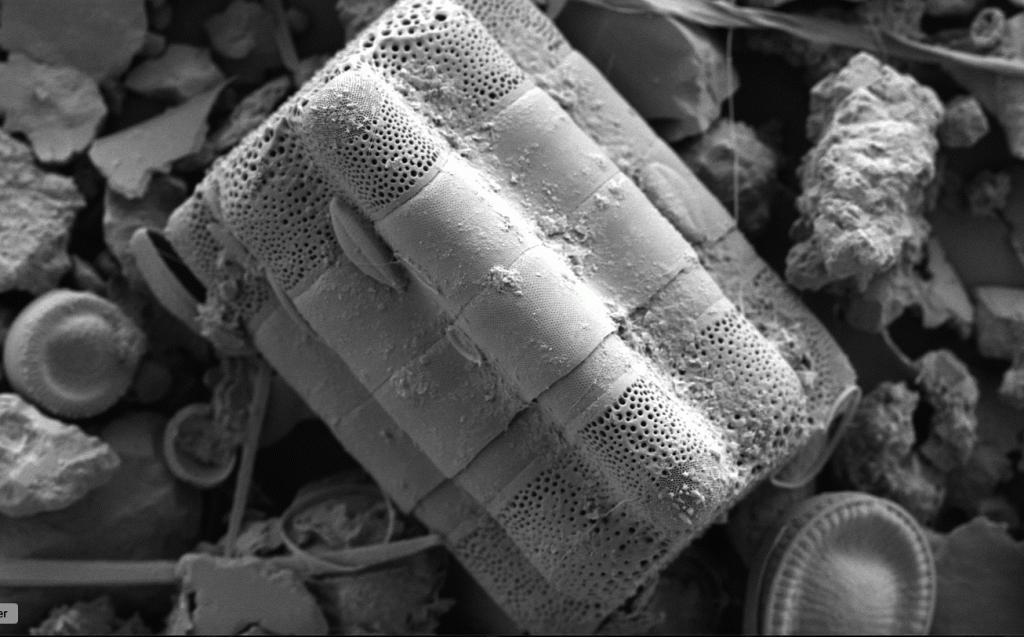
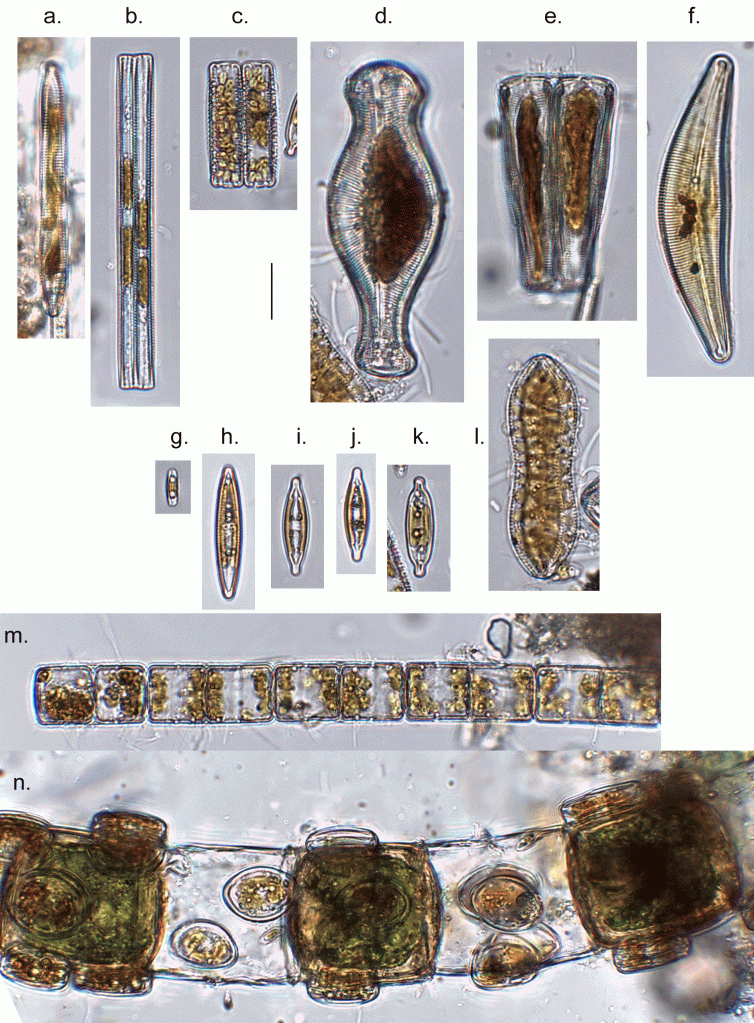
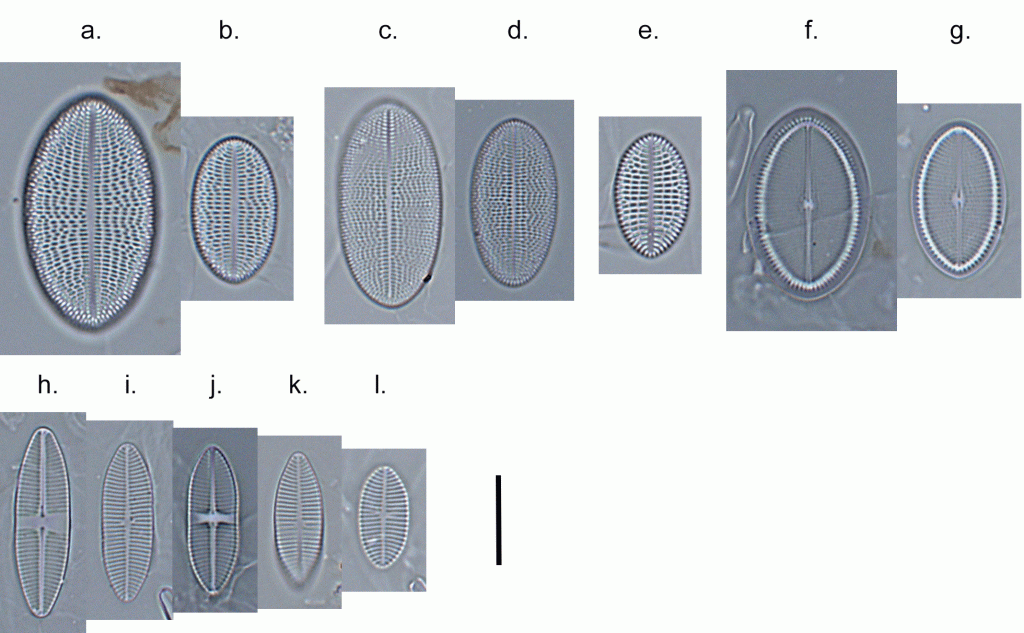
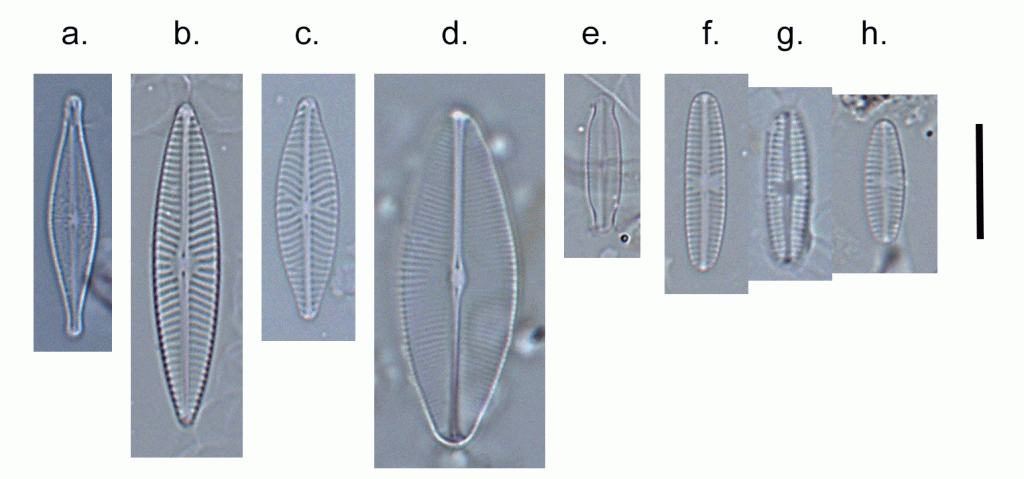
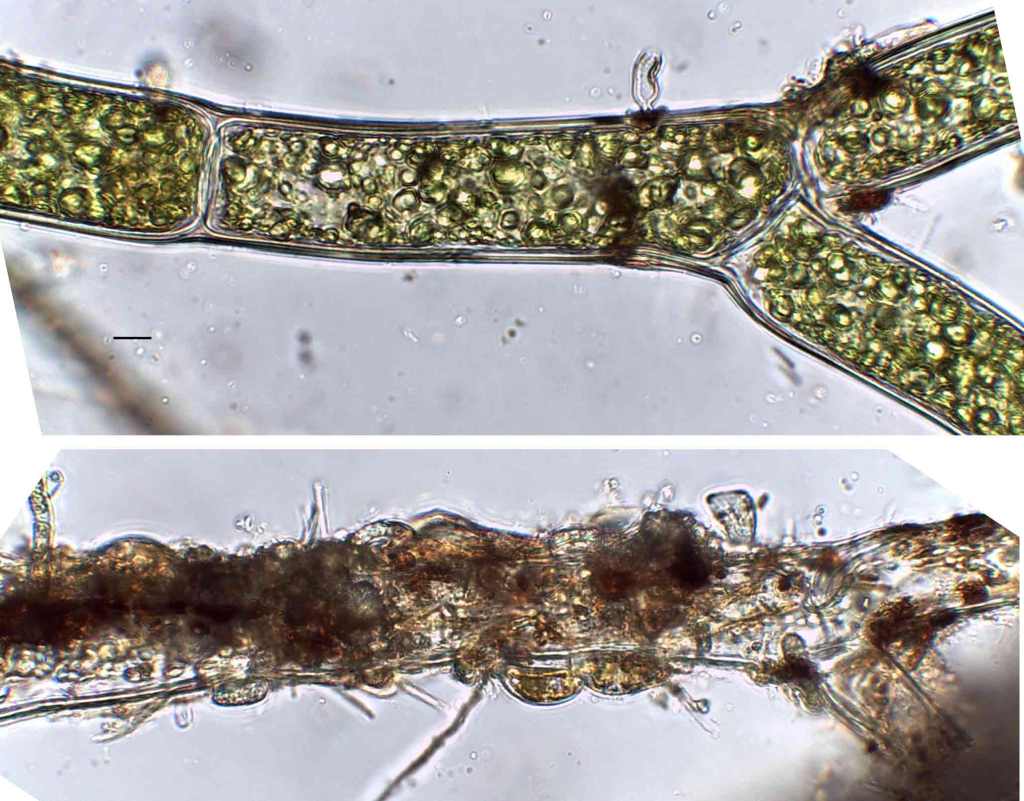
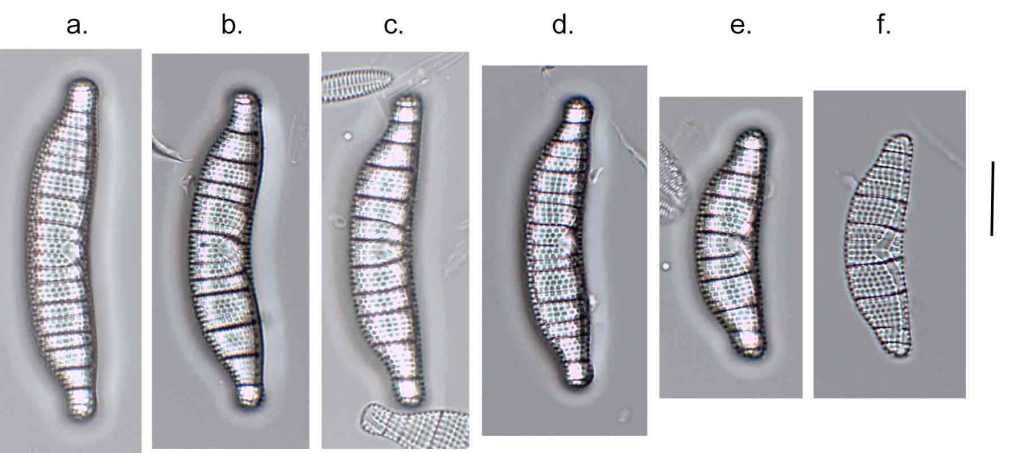
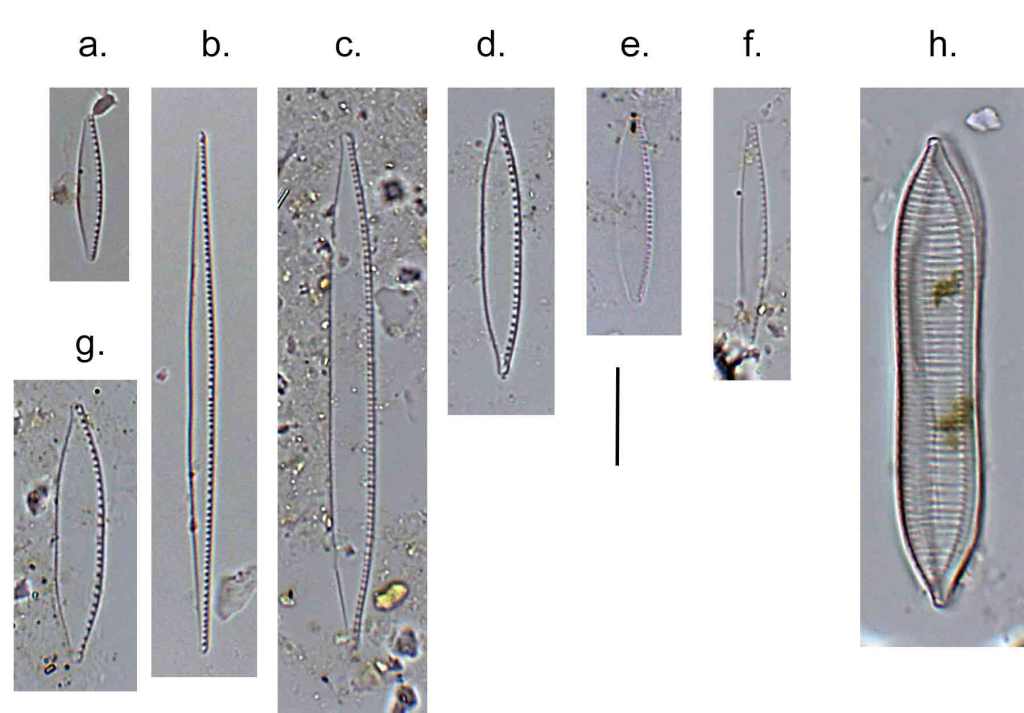
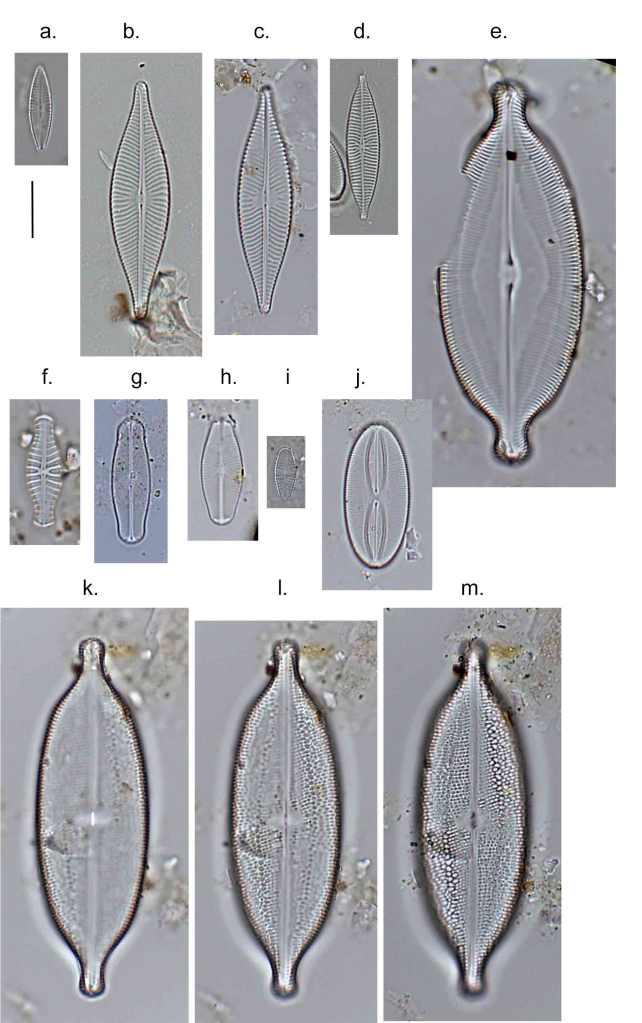
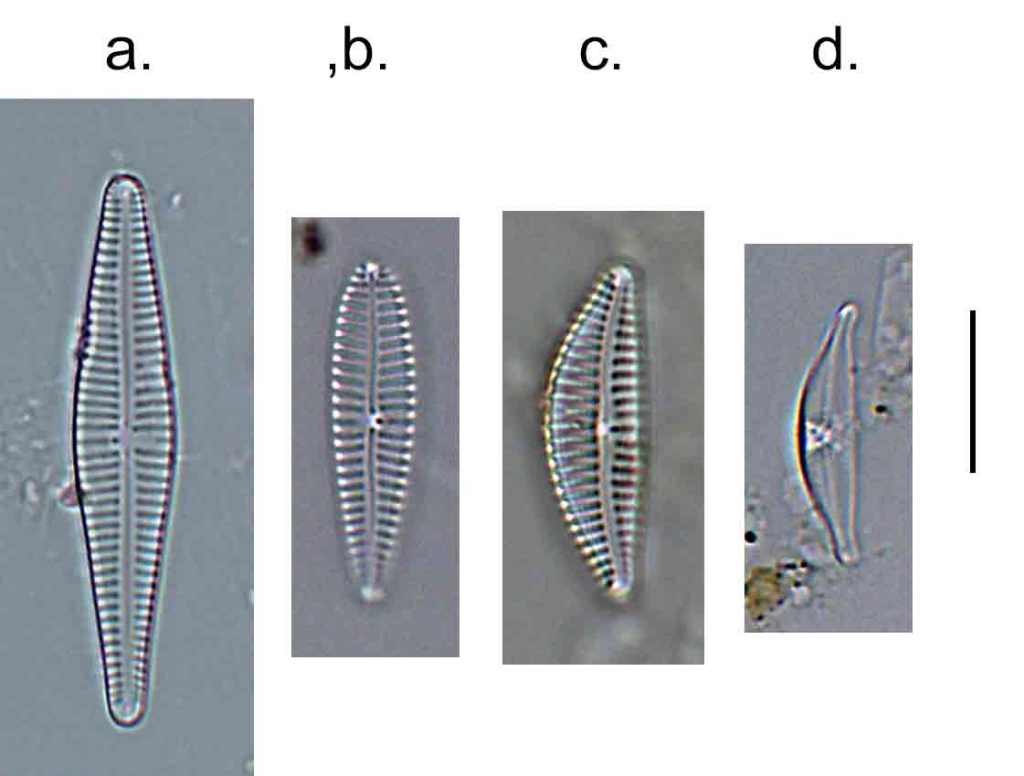
Few objects are more beautiful than the minute siliceous cases of the diatomaceae: were these created that they may be examined and admired under the higher powers of the microscope? The beauty in this latter case, and in many others, is apparently wholly due to symmetry of growth.
Charles Darwin (1859) On the Origin of Species
Over the past couple of years I’ve written several posts giving overviews of the major groups of freshwater algae (most recently “Origin story …” and “Unlikely bedfellows …”). I know that the of freshwater algae can be very confusing for outsiders, and I thought that sketching out some “family trees” might help folk work out how all these disparate groups link together. The one group that seem to have slipped through the net, however, are the diatoms, the group which is the main focus of my professional life.
That might be because I’m too close to the animated discussions that sometimes erupt around diatom systematics. Those discussions, in turn, point to an absence of unambiguous evidence concerning the origins of the major groups. David Williams and Pat Kociolek wrote in 2007 that “… a natural classification is still a long way off” and that statement still holds in 2022. As a result, what follows is a “work in progress” that should, at least, help ecologists see how the many different types of diatoms they encounter fit into a bigger picture.

Broadly speaking, classifications of diatoms can be divided into three phases:
- the era when classifications were based what you could see with a light microscopy;
- the era when classifications were based on what you could see with light and electron microscopy; and,
- the era when classifications were informed by molecular genetics, as well as by light and electron microscopy.
Whilst the first era extended from the invention of microscopes in the 17th century to the late sixties, the second and third eras have both been compressed into the last half century, the “early adopters” of molecular approaches rubbing up against conservative-minded scientists still largely reliant on light and electron microscopy.
When I started, the standard work was Fredrich Hustedt’s 1930 Süsswasserflora von Mitteleuropa, which treated diatoms as a class, divided into two orders, the centrics (diatoms with at least one plane of radial symmetry) and the pennates (diatoms with at least one plane of longitudinal symmetry). The pennate diatoms were, in turn, divided into those with and without raphes (the Araphidinae and Raphidineae respectively). Hustedt was not the originator of this classification, but his works informed so much taxonomy in the middle and later twentieth centuries, that it is his name that is most associated with it. Indeed, it was still the prevalent classification when Krammer and Lange-Bertalot started their revision of the Süsswasserflora in 1986.
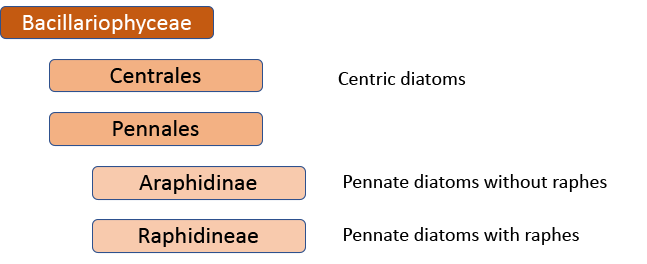
In 1990, however, Frank Round, Dick Crawford and David Mann produced a new classification, drawing much more on information from electron microscopy than Hustedt was able to do. The end result of this classification is much the same as before – with centrics, araphids and raphids separated – but the two groups of pennate diatoms were now of equivalent rank to the centric diatoms. Note, too, that diatoms as a whole have been promoted to a division rather than a class. This juggling of taxonomic ranks might seem like an unnecessary complication for end-users, but it is absolutely necessary if the end-result is a “natural classification”, as referred to above. Putting raphids and araphids as subgroups within a single pennate group implies that all pennate diatoms shared the same ancestor, regardless of whether or not they have a raphe. The Round/Crawford/Mann classification, by contrast, suggests that raphid and araphid pennates might be two separate lineages without a common ancestor.
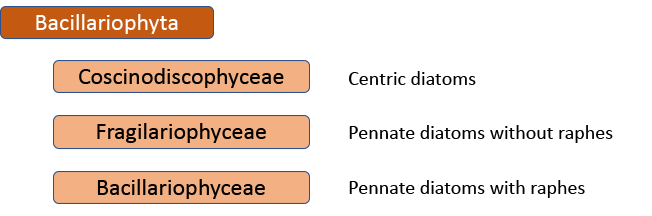
The first big impact of molecular genetics, however, was on the centric diatoms, hitherto thought of as a natural group. Linda Medlin and Irena Kaczmarska broke the diatoms into two subphyla, with the centric diatoms now divided between these. The Coscinodicophytina includes common freshwater diatoms such as Melosira varians (see “Some like it hot …”) whilst other common freshwater centrics such as Cyclotella and Stephanodiscus are in the class Mediophyceae within the Bacillariophytina, implying a closer relationship to pennate diatoms than hitherto suspected. The Mediophyceae, whilst common in freshwaters, tend to be planktonic rather than benthic, so I have never really given them the attention that they deserve in this blog. The pennates, meanwhile, are back as a single group, the Bacillariophyceae, with both araphids and raphids combined.
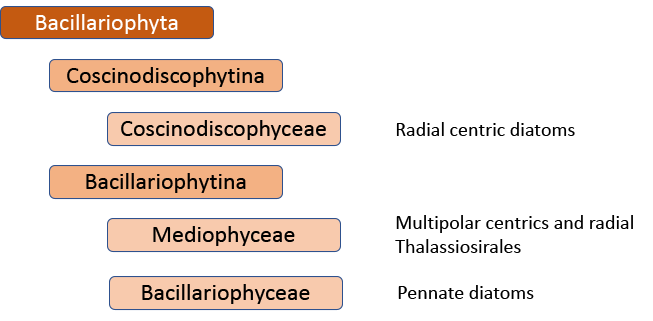
That’s almost the end of the story, insofar as the Medlin/Kaczmarska approach is used by AlgaeBase, the website that summarises taxonomic knowledge of the algae. However, further work has shown that this is still not a “natural” classification. That would mean yet another shake-up, probably with at least nine separate, albeit “natural”, groups (see Theriot et al. in reference list). There is now a very clear tension between those who believe that this is the best way forward in the long term and those who believe that minimizing the overall disruption experienced by end-users of taxonomic processes is also a consideration.
Interestingly, that final point may, itself, become the reason why we need a natural classification in the future. At present (and at risk of upsetting systematists), higher classification provides a useful indexing system that means I can pluck a book off my shelves, and flick through to find the appropriate pages, when I am trying to identify a diatom. All the older systems work just fine at that level. The problem comes when we are using metabarcoding to identify diatoms. Because our barcode reference libraries are far from complete, we cannot assign every sequence in a sample to an appropriate species. In such cases, it would be good to assign it to a higher taxon and, for this to work, our reference libraries need to be arranged around a meaningful hierarchy, and systems that are adequate for a pragmatic light microscopist will cause problems for the logical decision-making processes that are used in bioinformatics routines.
I’ve long argued that the “craft” of identifying diatoms is different to the science of taxonomy. I could go further: taxonomists have, in recent years, tended to be more concerned about cataloguing variation within species and genera (discovering a lot more diversity than hitherto expected in the process) than with understanding the interrelationships amongst these groups. However, we may now be closing the circle: “craft” of identifying diatoms got along fine with just occasional nods to systematics for a long time. The brave new world of metabarcoding might mean that systematics is, once again, recognised as the important discipline that it should be.
References
Adl., S., Bass, D., Lane, C.E., Lukeš, J. et al. (2018). Revisions to the classification, nomenclature, and diversity of eukaryotes. Journal of Eukaryotic Microbiology 66: 4-119.
https://doi.org/10.1111/jeu.12691
Medlin, L.K. & Kaczmarska, I. (2004). Evolution of the diatoms: V. morphological and cytological support for the major clades and a taxonomic revision. Phycologia 43: 245-270.
Round, F.E., Crawford, R.M. & Mann, D.G. (1990). The Diatoms: Biology and Morphology of the Genera. Cambridge University Press, Cambridge.
Theriot, E.C., Ashworth, M.P., Nakov, T., Ruck, E. & Jansen, R.K. (2015). Dissecting signal and noise in diatom chloroplast protein encoding genes with phylogenetic information processing. Molecular Phylogenetics and Evolution 89: 28-36.
Williams, D.M. & Kociolek, J.P. (2007). Pursuit of a natural classification of diatoms: history, monophyly and the rejection of paraphyletic taxa. European Journal of Phycology 42: 313-319.
Some other highlights from this week:
Wrote this whilst listening to: Wet Leg and caroline’s eponymous debut albums plus Father John Misty’s Chloë and the Next 20th Century.
Currently reading: Colm Tóibín’s The Magician, a novel about the life of Thomas Mann
Cultural highlight: first visit to the threatre since the start of the pandemic. Went to see Red Ellen – about the life of Labour politician Ellen Wilkinson – at Newcastle Playhouse.
Culinary highlight: “MSC carbonara”. Recipe: buy seafood which has a blue Marine Stewardship Council logo on it, and make a carbonara from it. This one was made from prawns, and enhanced by a pinch of chilli flakes and some lemon juice. The next one will be made from something else.