When I’m teaching people to identify diatoms, I start by telling them that there are two groups: the centric diatoms, characterised by at least one plane of radial symmetry, and the pennate diatoms, which have only longitudinal symmetry. This is a good place for a beginner to start but reality is, inevitably, more complicated. The two diatoms I describe in the previous post are, for example, both centric diatoms but it is difficult to discern this from the photograph, because their side views do not suggest any radial symmetry. But, as I’ve said many times before, taxonomy and identification require very different approaches.
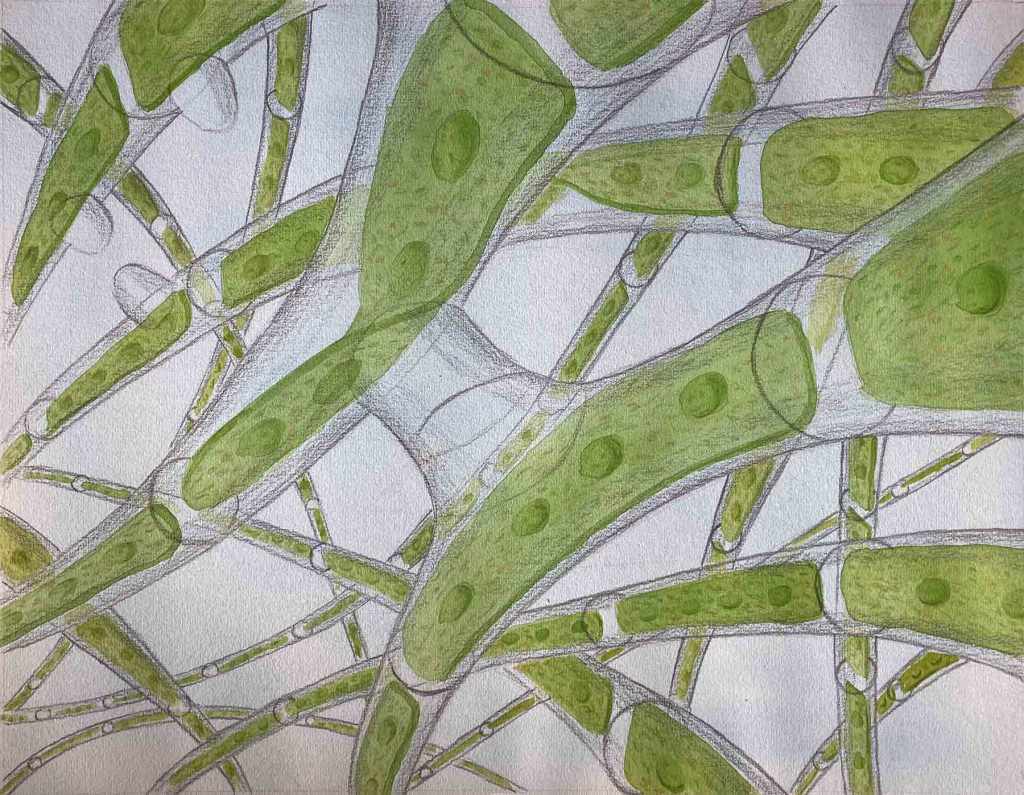
The basic centric/pennate dichotomy, however, is not just about outline, however convenient this is as a teaching aid for beginners. Centric diatoms have a different mode of reproduction (oogamous) compared to pennates (isogamous) and also typically have many discoid plastids rather than a few plate-like ones. I’ve illustrated one of the diatoms we saw at Deptford – Hydrosera triquetra – in the illustration at the top of the post, in order to delve deeper into this topic of shape as an identification aid.
The first thing we notice is that the valve face is not circular. The literature describes it as “triangular” but, in reality, the shape is closer in shape to a star-of-David. You could, however, take a pair of compasses and draw a circle that connected all of the valve angles, so you can see how this may be related to the more obviously circular diatoms. The valve face is covered with a seemingly random array of coarse pores apart from the ends of three of the valve angles (termed “pseudocelli”) which appear to be plain, but which are, in reality, a mesh of very fine pores through which mucilage is secreted. This allows the cells to join together to form chains, or to attach to surfaces.
Hydrosera triquetra is related to the other diatom I illustrated in the previous post – Biddulphia pulchella but whilst I can just about persuade you that Hydrosera is related to centric diatoms, it is harder to do this for Biddulphia, which is more obviously lanceolate in outline. It also has psedocelli, which hints at a relationship with Hydrosera, and also has many small discoid chloroplasts (hinting at a relationship with the wider centric diatoms) but it sure ain’t round, by anyone’s definition.
The efforts of many taxonomists to shoehorn Biddulphia into the centric diatoms recalls Thomas Kuhn’s observations in The Structure of Scientific Revolutions – scientists approach data with strong preconceptions and outliers to the prevailing wisdom require ever more elaborate justifications to fit the pattern, something Kuhn described as “puzzle solving”. Eventually, someone recognises that “normal science” is no longer viable, and proposes a new theory – a so-called “paradigm shift” (a phrase first used by Kuhn).
In the case of Biddulphia and Hydrosera, the paradigm shift came in a 2004 paper by Linda Medlin and Irena Kaczmarscka who proposed a new class, Mediophyceae, for these “multipolar centrics” based on molecular sequence data, and that has now become the established classification (see “Who do you think you are?”). Or, to use the language of Kuhn, we have re-entered a phase of “normal science”, at least until the next revolutionary biologist comes along with a better idea …
My picture was supposed to show Hydrosera triquetra growing on a vertical wall in Deptford Creek (with the bridge carrying the Dockland Light Railway over the river just visible in the background). There are eight orders of magnitude difference between the Hydrosera cells (which are relatively chunky by the standards of diatoms) and the DLR bridge – which is a depth of field way beyond any camera could achieve. Less impressive, from my point of view, is the appearance of a few mutant Hydrosera cells with five, rather than six, poles. I finished the picture in rather a hurry as I wanted to post this before we headed off on holiday. Sorry.
References
Ashworth, M. P., Nakov, T., & Theriot, E. C. (2013). Revisiting Ross and Sims (1971): toward a molecular phylogeny of the Biddulphiaceae and Eupodiscaceae (Bacillariophyceae). Journal of Phycology 49: 1207-1222.
Kuhn, T. (1962). The Structure of Scientific Revolutions. University of Chicago Press.
Medlin, L. K., & Kaczmarska, I. (2004). Evolution of the diatoms: V. Morphological and cytological support for the major clades and a taxonomic revision. Phycologia 43: 245-270.
Some other highlights from this week:
Wrote this whilst listening to: Marika Hackman, following her gig at the Cluny in Newcastle.
Currently reading: The Story of Art Without Men by Katy Hessel. My daughter thought I needed educating.
Cultural highlight: Marika Hackman in a packed Cluny. Can’t help wondering why she’s not better known.
Culinary highlight: immediately prior to the gig, we ate at the Ship Inn, just across the road, which has all the attributes of a traditional Newcastle pub with football on big screens and “pub grub” on the menu, only the whole menu is vegan. We ate their take on fish and chips – tofu wrapped in seaweed and then battered and deep fried – which rather proved my point that fish is algae-flavoured protein.