Five weeks have elapsed since my last post – the longest gap since I have started this blog. I think of it as a brief sabbatical and am returning revitalised and reenergised, I hope, just as the country starts to emerge from what seems like a long, damp and dreary winter. I got in the car, turned on a podcast and set off in search of a different type of river to any that I have previously described in Microscopes and Monsters.
My destination is a village called Darton, just north of Barnsley, in South Yorkshire, once the heart of the South Yorkshire coalfield, though evidence of this former industrial heritage is hard to find. In the past, many of the men in this village worked at Wooley Colliery, as did, at one point, Barnsley-born Arthur Scargill, leader of the National Mineworkers Union during the 1984-85 Miner’s Strike. But the colliery closed in 1987 and the surface structures have since been demolished.
Underground, however, there are still hundreds of kilometres of abandoned workings. When the mine was active, water that found its way into these workings had to be pumped to the surface to keep the mines from flooding. As it flowed over the exposed coal seams, it became contaminated and the pumped minewater created major pollution problems in local rivers. Pumping had to continue after the mines closed otherwise the water would build up and eventually burst out of one of the abandoned shafts, with devastating consequences for the rivers downstream, as happened at Wheal Jane Mine in Cornwall in 1992. So the River Dearne, the stream that flows through Darton, continued to receive pumped minewater long after Whalley Mine itself had closed down.
I last encountered the Dearne about ten years ago, when I was looking for an example of a saline river for another project. The salinity occurs when minewater comes into contact with minerals deep underground, which then, due to the absence of oxygen and (often) low pH, dissolve resulting in brines that can be more saline than seawater. After treatment and dilution, the Dearne’s water was not as salty as seawater, but there was at this time still a detectable “brackish” effect. I thought it might be interesting to return to this location as a contrast to my recent excursions to Deptford Creek but, as the graph below shows, recent improvements in treatment mean that the salinity (as measured by conductivity) is now below the level where any effects are likely to be seen. The conductivity on the day that I visited was, however, 1856 mS cm-1, which is higher than these recent measurements, and in the range where some ecological impact might be expected. I should point out that the Environment Agency does not include salinity as part of its formal status assessment of rivers, so high conductivity will not, by itself, influence their classification results.
Conductivity in the River Dearne at Darton from 2013 to 2020. The dashed lines are approximate levels where chronic (lower) and acute (upper) effects of salinity are likely to be experienced. They are based on analyses in Kelly et al. (2024). The photograph at the top of the post shows the River Dearne at Darton, photographed in May 2024.
That’s not the end of the story because this just means that the Dearne is yet another polluted lowland polluted river, receiving inputs from small sewage works as well as the pumped minewater and agricultural runoff. The Environment Agency classifies the biology here as “poor” and I am not going to disagree based on what I could see. The river bed was smothered with Cladophora glomerata and most of the diatoms that I saw through my microscope belonged to species that could tolerate elevated nutrient concentrations. Several of them also thrived at Deptford Creek, interestingly – Navicula lanceolata and Rhoicosphenia abbreviata, for example (see “Floundering around in Deptford Creek”). They are freshwater species that can tolerate some salinity, rather than brackish species that can tolerate freshwater. I’m guessing that the conductivity here fluctuates a lot, depending on the relative balance of minewater and surface water and, on my visit, treated minewater formed a greater part of the total flow, leading to the high conductivity I measured.
Cladophora glomerata smothering a boulder in the River Dearne at Darton, May 2024.
In my first paragraph, I said that I had set off in search of a different type of river to any that I have previously described in Microscopes and Monsters. I found, instead, a river rather similar to many in my own area (also a former coal mining area). That was partly because the reason that the Dearne was special in the past was a pollution source whose treatment had been improved in recent years. I cannot complain: an interest in the impact of pollution usually comes from a desire to remove its effects. The conundrum is that this in turn makes it harder to understand the effects the more esoteric forms of pollution have on our landscapes. I failed in my objective but only, I guess, because a wider goal had been achieved.
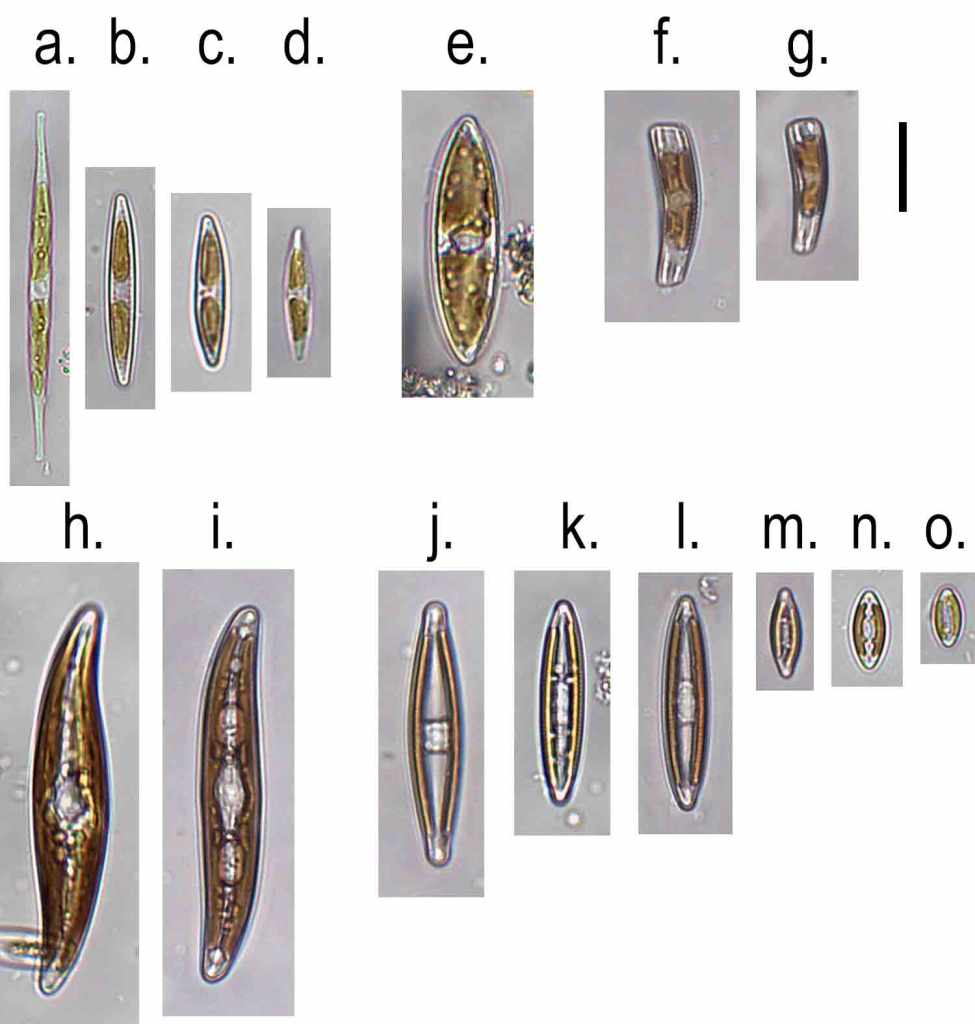
Diatoms from the River Dearne, May 2024. a. Diatoma vulgaris; b. Navicula lanceolata; c. Melosira varians; d. Rhoicosphenia abbreviata; e. Gyrosigma cf. acuminatum. Scale bar: 20 micrometres (= 1/50th of a millimetre).
In my first paragraph, I said that I had set off in search of a different type of river to any that I have previously described in Microscopes and Monsters. I found, instead, a river rather similar to many in my own area (also a former coal mining area). That was partly because the reason that the Dearne was special was a pollution source whose treatment had been improved in recent years. I cannot complain: an interest in the impact of pollution usually comes from a desire to remove its effects. The conundrum is that this in turn makes it harder to understand the effects the more esoteric forms of pollution have on our landscapes. I failed in my objective but only, I guess, because a wider goal had been achieved.
References
Kelly, M.G., Teixeira, H., Lyche Solheim, A., Free, G., Phillips, G., Salas Herrero, M.F., Kolada, A., Varbiro, G. & Poikane, S. (2024). Physico-chemical criteria to support Good Ecological Status in Europe. Publications Office of the European Union, Luxembourg. [https://publications.jrc.ec.europa.eu/repository/handle/JRC136407#:~:text=Physico%2Dchemical%20criteria%20to%20support%20Good%20Ecological%20Status%20in%20Europe,-2024Technical%20reports&text=This%20report%20summarises%20approaches%20to,%2C%20temperature%2C%20salinity%20and%20acidification.]
Laine, D. M. (1999). The treatment of pumped minewater at Woolley Colliery, West Yorkshire. Water and Environment Journal 13: 127-130.
Younger, P.L. (2000). The adoption and adaptation of passive treatment technologies for mine waters in the United Kingdom. Mine Water and the Environment 19: 84-97.
Some other highlights from this week:
Wrote this whilst listening to: Bop Til You Drop by Ry Cooder
Currently reading: Lamentation by C.J. Sansom who, sadly, died last week.
Cultural highlight: A performance by the Daniel Martinez Flamenco Company at Northern Stage in Newcastle.
Culinary highlight: some great memories of recent meals in Chengdu