Last week saw the second presentation of the FBA’s algae identification course for 2023, with another dozen keen participants being introduced to the intriguing world of freshwater algae. The week starts with sampling in the vicinity of the FBA’s laboratory, first at Windermere itself (see “A hitchhiker’s guide to phytoplankton …”) and then, the following morning, at other sites in south Cumbria. I usually join the group heading to Cunsey Beck, which flows from Esthwaite Water to Windermere but heavy overnight rain meant that river levels were too high for safe wading. Instead, we joined Allan Pentecost on a trip to White Scar Quarry, right at the south-eastern tip of the Lake District. It is an area that Allan knows very well and I always enjoy visiting with him because I learn a lot (see, for example, “Love and sex in a tufa-forming stream …”).
Just at the edge of the quarry, a small seepage flows across a wide bedding plane and, Allan pointed out to us, an impressive range of Cyanobacteria, green algae and moss co-existed side-by-side. If you look at the photograph at the top of the post, you can see the seepage emptying onto the bedding plane just above the “d.”. At low flow conditions, there is just a gentle trickle of water running in a narrow band approximately at the centre of the picture. However, when there is more water coming down the quarry, then the water spreads out further and is also augmented by rainfall landing directly on the bedding plane. The result is a zonation, roughly akin to what would be seen on a rocky shore, albeit not for seaweeds.
Following this from the left we have:
- The driest area, dominated by Nostoc commune whose ecosystem-building capabilities in otherwise adverse environments I’ve described in earlier posts (see “Landscape architects …” for the most recent of these);
- An only occasionally wetted area dominated by dark brown mats of Scytonema. The colonies described in “Poking around amongst sheep’s droppings …” came from very close to here and will give you some idea of what to expect;
- A slightly wetter area has reddish mats of filamentous cyanobacteria. Schizothrix, Phormidium and Homoeothrix all feature here. An example of a Phormidium growing at the air-water interface (and, therefore, presumably tolerant of desiccation) is described in “Fieldwork notes, August 2021”)
- The central zone is almost permanently wet and here there is a distinct growth of the green alga;
Mougeotia, a very common alga in this part of the world. Recent posts which mention this genus include “Something, somewhere, just for a moment …” and “The man who stares at algae …” (both, incidentally, describe the interplay between green algae and Cyanobacteria in Lake District streams).
From here, the sequence is reversed except, at the right-hand side of the picture frame (e.) we did not see more Nostoc commune but, instead, patches of Rivularia haematites and bright patches of the moss Philonotis fontana. I last wrote about Rivularia in “Building landscapes …”, based on another excursion that Allan led, this time in the Malham area of Yorkshire rather than in the Lake District. I wonder, in retrospect, if the moss is, itself, a result of the ecosystem-building properties of Nostoc that I mentioned above and also wrote about in “How to make an ecosystem (2)”. If I had teased apart those Philonotis clumps, would I have seen colonies of Nostoc lurking at the bottom, perhaps? We hear a lot about all the problems Cyanobacteria cause in the Lake District at the moment so it is useful, once in a while, to remind ourselves that Cyanobacteria helped to build this wonderful landscape in the first place.
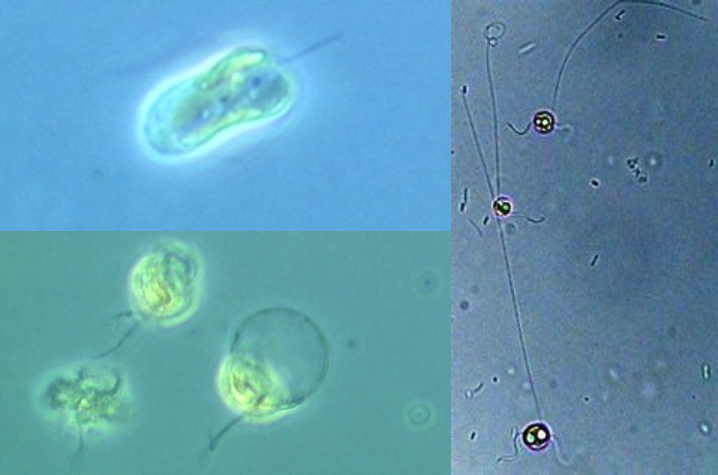
Microscopic views of the zonation at White Scar Quarry, Cumbria, September 2023. a. Nostoc commune; b. Rivularia haematites; c. Phormidium sp.; d. Mougeotia sp. No scale bars, I’m afraid, as the images were grabbed ad hoc while I was teaching, but other posts referenced should all have indications of size.
Some other highlights from this week:
Wrote this whilst listening to: Africa Express Presents … The Orchestra of Syrian Musicians and Guests.
Currently reading: Maggie O’Farrell’s The Wedding Portrait, set in Renaissance Italy.
Cultural highlight: Exquisite Korean-American film Past Lives directed by Celine Song. A love story mostly set against the Manhattan skyline.
Culinary highlight: It may seem like a minor achievement, but I made the best shortbread of my life this week, following the Ayrshire Shortbread recipe in Cerys Matthew’s cookbook, Where the Wild Cooks Go …. The vegan haggis that I made from her recipe also went down well a couple of weeks ago.
Patches of Philonotis fontana on the bedding plane at White Scar Quarry, September 2023.